Cholera pathogenesis

The PDB code links will take you to the PDB entry with links to the publication
Pathogenicity islands

Several hypothetical proteins are present in only toxigenic strains of V. cholerae. A structural proteomics study is aimed at discovering their function. VC1804 & VC1805 are homologues in VP-2, with two paralogues VC0508 & VC0509 in the VSP-I island. They are structurally similar to the human mitochondrial protein p32. This is a collaboration with Fidelma Boyd, U. of Delaware, USA.
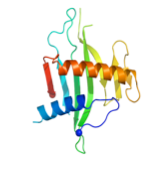


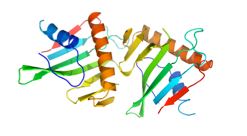
VC1805 VC0508 VC0509

Quorum sensing
Comparison of VC1805 with human p32 is shown on the right. p32 has an N-terminal helix and longer C-terminal helix, both involved in trimer formation. Apart from that, the topology is the same, as is a predominantly negative charge. VC1805. like p32, binds to C1q of the complement system, but its function, and why 4 copies of this protein gives an advantage to V. cholerae is yet to be established.
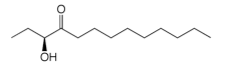
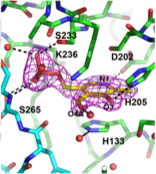
CAI-1 is the major autoinducer produced by V. cholerae. It is synthesised by CqsA and detected by the CqsS receptor. CqSA belongs to the PLP-dependent alpha-oxoamine synthetase family that typically condense an amino acid with an acyl-CoA substrate. We have determined the structure of CqsA alone, in complex with PLP, and have trapped an external aldimine complex that suggests a mechanism.

CqsA
CAI-1
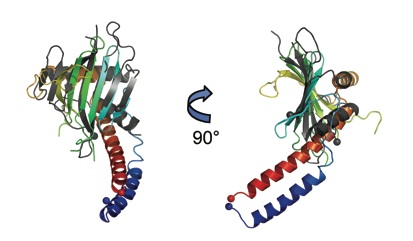
Ferric uptake regulator


The ferric uptake regulator (Fur) acts as both a repressor and activator of numerous genes involved in maintaining iron homeostasis in bacteria. It has also been demonstrated in V. cholerae that Fur plays an additional role in pathogenesis, and this opens up the potential of Fur as a drug target for cholera. Comparison with P. aeruginosa Fur shows very different orientation of DNA binding domains.






