Guidance Notes for the Safe Storage and Handling of Cryogenic Materials (December 2002)
Contents
1. University of St Andrews Guidance
1.1 Introduction
1.2 Implementation
1.3 Duties and Responsibilities
1.4 Relevant Legislation
2. Properties of Cryogenic Materials
3. Action Plan for Liquid Cryogenics
4. Hazards
4.1 Cold Burns, Frostbite and Hypothermia
4.2 Oxygen Deficiency and Asphyxiation
4.3 Oxygen Enrichment
4.4 Pressurisation and Explosion
4.5 Damage to Equipment
4.6 Flammable Gas - Hydrogen
4.7 Specific Guidance for Liquid Helium User
5. Safety and the Use of Cryogenic Liquids - Guidance
5.1 Protective Clothing
5.2 Training
5.3 Tips on Handling Cryogenic Liquids - Do It With Care!
5.4 Spillage Procedures and Notification
5.5 Disposal
5.6 First Aid
5.7 Storage
5.8 Transportation
ANNEXE I Physiological Effects and Recommended Exposure Limits of Nitrogen
ANNEXE II Guidance for Assessment of Ventilation Requirements
ANNEXE III Method of Calculating the Potential Oxygen Depletion in a Room due to Liquid Nitrogen Filling and Spillage
ANNEXE IV Oxygen Depletion Example
ANNEXE V Cryogenic Storage Checklist
ANNEXE VI Small Volume Users Checklist
ANNEXE VII Transport Emergency Card
ANNEXE VIII Emergency Action Plan
NOTE: Annexes I - IV are extracted from British Compressed Gases Association (BCGA) Code of Practice CP30 - 'The Safe Use of Liquid Nitrogen Dewars up to 50 Litres' and are published with the permission of the BCGA.
1. Guidance on the Storage and Handling of Cryogenic Materials
1.1 Introduction
Cryogenic materials are very cold substances used in a wide variety of processes, including cooling probes and preserving biological samples. There are certain hazards associated with the use of cryogenic materials such as cold burns, explosion and asphyxiation. It is the aim of the University to ensure that all persons handling cryogenic materials are fully trained in their use and not exposed to danger in accordance with the relevant legislation and guidance in this code.
This document sets out the University of St Andrews guidance on the storage and handling of cryogenic materials, identifies the associated hazards and gives the basic safety precautions that must be adopted.
1.2 Implementation
The Head of School/Unit is responsible for the implementation of this guidance in the areas under their control. Simply issuing this guidance to the concerned parties does not constitute implementation. Compliance should be achieved through the dissemination of information and the provision of appropriate training to all relevant persons.
1.3 Duties and Responsibilities
The Head of School/Unit shall ensure that:
- There is a training programme on the use and handling of cryogenics;
- The necessary equipment and personal protective equipment (PPE) is provided;
- Safe storage facilities for cryogenic materials are provided and maintained;
- A self inspection programme covering the use and storage of cryogenic materials is implemented;
- There are written procedures for the use and handling of cryogenic materials;
- There are written emergency protocols;
- The University Safety Adviser is notified of all bulk storage and dispensing areas;
- Sufficient resources are allocated to cover the above items.
Supervisors/Principal Investigators shall ensure that:
- Suitable and sufficient risk assessment of the use of cryogenic materials are conducted;
- There is provision of appropriate PPE;
- There is adequate training and, where necessary, supervision;
- Training records are maintained for each individual.
Individual Users (All Staff and Students) shall ensure that:
- They take reasonable care of themselves and others affected by their actions;
- Risk assessments are complied with;
- They wear the PPE provided when handling cryogenic materials;
- They follow instructions on handling cryogenic materials as described during training
1.4 Relevant Legislation
The following legislation applies to the handling, storage and use of cryogenic materials.
- The Pressure Systems Safety Regulations (2000);
- The Pressure Equipment Regulations (1999);
- The Manual Handling Operation Regulations (1992);
- The Confined Spaces Regulations (1997);
- The Personal Protective Equipment (PPE) Regulations (1992);
- The Management of Health and Safety at Work Regulations (1999);
- The Carriage of Dangerous Goods by Road Regulations (1996);
- The Provision and Use of Work Equipment Regulations (1998)
Each set of Regulations is complemented by an Approved Code of Practice, which states how to implement the Regulations. Further information can be found in the following leaflets and on the BCGA web-site http://www.bcga.co.uk/first.htm
- Storage of Packaged Dangerous Substances HS(G)71, HSE 1992, ISBN 0-1188-5989-7;
- "Safety of Pressure Systems" - Pressure Systems Safety Regulations 2000 and Approved Code of Practice L122 HSE 2000, ISBN 0-7176-1767-X;
- Approved Vehicle Requirements (Second Edition) - Carriage of Dangerous Goods by Road Regulations 1996 L89, HSC 1999, ISBN 0-7176-1680-0;
- Manual Handling - Manual Handling Operations Regulations 1992 - Guidance on Regulations L23, HSE 1998, ISBN 0-7176-2415-3;
- Safe Work in Confined Spaces - Approved Code of Practice, Regulations and Guidance - Confined Spaces Regulations 1997 L101 HSC 1997, ISBN 0-7176-1405-0.
2. Properties of Cryogenic Materials
Cryogenic liquids are liquids that exist between -66ºC and -266ºC. The most common cryogens used in the laboratory are liquid nitrogen, liquid helium and solid carbon dioxide (dry ice), although there are others including liquid oxygen and liquid argon.
| Property | Oxygen (02) | Nitrogen (N2) | Argon (Ar) | Helium (He) | Carbon Dioxide (C02) |
| Molecular weight | 32 | 28 | 40 | 4 | 44 |
| Colour of gas | None | None | None | None | None |
| Colour of liquid |
Light blue |
None | None | None | None |
| Boiling point (ºC) at atmospheric pressure | -183 | -196 | -186 | -269 | -78.5 (Sublimes) |
| Ratio of volume gas to liquid at 15ºC 101.3kPa | 842 | 682 | 822 | 738 | 845 (Solid) |
| Explosive/fire danger | Yes | No | No | No | No |
| Toxic | No | No | No | No | Mildly |
Due to the great expansion ratio of cryogenic gases, a spillage can result in significant oxygen depletion within the room, which may be life threatening.
Guidance for the assessment of ventilation requirements is given in Annexe II.
A method for calculating the potential oxygen depletion in a room due to liquid nitrogen filling and spillage is given in Annexe III. A worked example is given in Annexe IV.
3. Action Plan for Liquid Cryogenics
A copy of the action plan can be downloaded: Action Plan for Liquid Cryogenics (RTF, 13 KB)

4. Hazards
4.1 Cold burns, frostbite and hypothermia
- Contact of the skin with cryogenic liquids (or even cold gas) can cause severe cryogenic burns; the tissue damage that results is similar to that caused by frost bite or thermal burns. While the cold itself can reduce the feeling of pain, the subsequent thawing of tissue can cause intense pain.
- Contact with non-insulated parts or equipment or vessels containing cryogenic liquids can produce similar damage. Unprotected parts of the skin may stick to low-temperature surfaces and flesh may be torn upon removal.
- Inhalation of cold vapour can cause damage to the lungs and may trigger an asthma attack in susceptible individuals.
- Hypothermia is risk due to the low temperatures arising from the proximity of cryogenic liquids. Risk is dependent upon the length of exposure, the atmospheric temperature and the individual; those exposed for prolonged periods should be warmly clothed.
- The low viscosity of cryogenic liquids means that they will penetrate woven or other porous clothing materials much faster than, for example, water.
N.B. See Section 5.6 for First Aid
4.2 Oxygen deficiency and asphyxiation
Whilst not toxic themselves (excepting CO2 which is mildly toxic), the cryogenic gases are capable of causing asphyxiation by displacing the air necessary to support life.
A reduction in atmospheric oxygen results in loss of mental alertness and distortion of judgement and performance. This occurs in a relatively short time period and without the person being aware it is happening.
An oxygen shift as low as 3% below 20.9% (normal air concentration) is potentially dangerous and atmospheres containing less than 10% oxygen can be fatal.
Upon evaporation, the volume of cryogenic liquid expands approximately 700 - 900 times its volume in the gaseous form. If this occurs in a room that is inadequately ventilated, atmospheric oxygen will be displaced. This will result in the oxygen content of the air being reduced to such an extent that it will not sustain life.
The onset of oxygen deficiency problems is often not apparent to the individual involved as there are few warning signs. In going to assist unconscious colleagues, rescuers themselves are often overcome by the lack of oxygen, resulting in further fatalities.
N.B. If you find someone unconscious and suspecting asphyxiation, the alarm should be raised and you should activate the Emergency Action Plan for a Major Release of Toxic Gas, Low Temperature Liquefied Gas or Major Spillage of Hazardous Substance (see Annexe VIII).
Emergency action and rescue should be well planned in advance. Rescue should only be attempted by those trained in the use and of wearing breathing apparatus and familiar with confined space entry procedures - see HSE Guidance Note GS5 - "Entry into Confined Spaces". At the University of St Andrews the Fife Fire Brigade must be contacted if such a rescue is required.
4.3 Oxygen Enrichment
Although itself not flammable oxygen, when present in higher concentrations, can significantly increase the chance of fire or an explosion.
The boiling point of oxygen is above those of nitrogen and helium. In closed systems (such as cold traps cooled with liquid nitrogen) these liquids can cause oxygen to condense on their surface (resulting in a bluish liquid on the surface). This can lead to the ignition of normally non-combustible materials and the flammability limits of flammable gases and vapours are widened. Oil and grease may spontaneously ignite and as such should not be used where oxygen enrichment may occur.
4.4 Pressurisation and Explosion
Cryogenic liquids vaporise with a volume change ratio of 700-900 and can thus cause violent changes in pressure, particularly if this occurs in a confined space. This in turn can result in an explosion. Vent systems must be in place to allow gas to escape from confined spaces. Pressurisation can occur due to the following:
- Ice forming on the venting tube, plugging it and preventing gas release;
- Damaged equipment resulting in cryogenic fluids leaking into small areas. Upon vaporisation the cryogenic liquid vaporises and causes pressure build up;
- Loss of vacuum inside a cryostat or dewar;
- If a liquid helium-cooled super-conducting magnet "quenches" (changes spontaneously from a super-conducting state to a normal state);
- Liquid nitrogen having permeated through sealed cryo-tubes containing samples which then return to room temperature;
- Direct contact of the cryogenic liquid with water in a tube results in rapid vaporisation of the cryogenic liquid and can cause the tube to explode.
4.5 Damage to Equipment
The very cold temperatures of cryogenic liquids can damage equipment and materials, which can result in danger.
- Spilled liquid nitrogen can crack tiles and damage flooring such as vinyl;
- Rubber tubing may become brittle and crack during use;
- Condensation of water around electrical cables may result in an electrical shock hazard.
4.6 Flammable Gas - Hydrogen
Hydrogen is extremely flammable and should be treated with extreme caution. Areas of use should be restricted, clearly marked and well ventilated. No naked flames, electrical ignition sources or potentially combustible materials should be allowed within the restricted area as any of these could result in an explosion if gas has escaped.
Liquid hydrogen can condense oxygen from air resulting in an explosion hazard. For this reason closed hydrogen systems should be used to prevent back-flow of air.
4.7 Specific Guidance for Liquid Helium Users
Only experienced and properly instructed people should handle liquid helium.
Before Using Liquid Helium:
a) Read the following guidelines
b) Know and understand the properties and hazards associated with it
c) Establish plans to cover any emergency situations
d) Understand your cryostat and its correct operation
If in doubt - ask your Supervisor!
Liquid Helium - Special Precautions
To know what precautions to take is to recognise that at 4º Kelvin, all other gases solidify. Therefore, helium systems and dewars must prevent back flow of air as this constitutes a major safety hazard.
Small volumes of liquid evaporate into large volumes of gas and must be allowed to vent safely, therefore:
- Always connect to helium recovery line and open appropriate vent valves;
- Never leave a dewar open to atmosphere;
- Use liquid helium only in well ventilated areas;
Dewars open to atmosphere for prolonged periods can cause "ice plugs" causing pressure build-up which can lead to over-pressurisation and potential catastrophic failure (explosion).
Liquid Helium - Safe Handling and Usage
a) Minimum protection recommended is:
i) Cryogenic gloves
ii) Face visor or safety glasses
b) Always inspect the dewar
High pressure in the dewar will be indicated by:
- an inflated rubber bladder when the connection valve is opened
OR
- a high pressure reading on the pressure gauge on dewar (if fitted)
Vent any gas slowly through the helium recovery line if found in this condition.
c) Never drop objects into the liquid
Beware of cold gas and rapidly boiling helium when lowering equipment at ambient temperature into the dewar. This operation must be carried out slowly to minimise boil off and potential cold vapour burn.
d) Never accompany a dewar in a lift
A sudden release of vapour in a confined space could be fatal!
e) Always use correct syphon and fittings
A vacuum insulated syphon is the only method of transferring helium from a dewar. It consists of a vacuum shielded tube, which dips below the liquid level in the dear. The dewar is then lightly pressurised which forces the liquid up and out through the syphon tube.
It is important the correct order of assembly of a dewar head syphon is followed to prevent leaks. Remove brass plug and lock nut used to seal dewar when not in use. Insert syphon 'slowly' and tighten brass flange with the lock nut to seal the "o" ring.
ENSURE ALL PRESSURE HAS BEEN REMOVED BEFORE PERFORMING THIS OPERATION
f) Always transfer liquid slowly
To prevent thermal shock, avoid high pressure build-up (back pressure). This also uses the helium most efficiently.
g) Never pressurise with gas other than helium
Pressurisation with a bladder is sufficient for most purposes and this is obtained by squeezing the bladder to create a slight over-pressure.
Do not use external regulated supplies unless competent to do so
h) Pre-cooling equipment
Any liquid nitrogen used to pre-cool liquid helium space in cryostats must be fully removed prior to adding liquid helium.
i) Purging
Purging of syphons and cryogenic equipment for liquid helium service should only be done with dry liquid helium gas.
j) Always thaw equipment with hot air
This is by far the quickest and safest method
Liquid Helium - Transfer Efficiency
a) Syphon and cryostat
Ensure good vacuum is maintained in both
b) Syphon and cryostat cool-down
Complete this operation slowly to prevent thermal shock or high back-pressure
c) Boil-off due to pressurisation gas (external supplies only)
Slowly apply the helium pressurisation gas as it is hot compared to the liquid
d) Depressurisation loss
Do not pressurise the dewar more than is necessary to perform the transfer and always try to fill in one operation.
Ice Plugs in Dewar Necks
On rare occasions an ice plug may form in the neck of the dewar. This must be dealt with quickly since pressure build-up is potentially dangerous. Take action as follows:
If you discover a dewar which has been left open to atmosphere for a period of time (e.g. via syphon entry port, helium recovery valve or bladder pressurisation valve):
1) Probe the inside of the dewar with helium dipstick to establish if it is clear and able to vent.
2) Report the event to your Supervisor, senior technical officer or Safety Co-ordinator
3) If the dewar is blocked or partially blocked:
i) clear laboratory of all personnel
ii) inform your immediate Supervisor
5. Safety and the Use of Cryogenic Liquids - Guidance
5.1 Protective Clothing
Personal protective equipment (PPE) must be worn when handling cryogenics. However, it is only there to prevent against accidental spillage, splashes, contact with cold surfaces and explosion risks. Further guidance on the selection, use and care of PPE is given in the University publication entitled "The Selection, Use and Maintenance of Personal Protective Equipment (PPE)".
PPE IS NOT DESIGNED TO WITHSTAND IMMERSION IN OR PROLONGEDCONTACT WITH CRYOGENIC LIQUIDS!
The following equipment must be worn when handling cryogenic materials:
Face shield - protect the users face and eyes against splashes
Gloves - must conform to BS EN 511 (Cold Protection). The gloves should either have been specifically designed for cryogenic handling with ribbed cuffs to prevent splashing into the glove or be loose fitting gauntlets that can easily be removed. The material should be rough to give good grip while handling and not increase the chance of spillage.
Aprons/Overalls - avoid woven materials if possible, if they are used it is essential to ensure they do not become saturated with cold liquid. Fastenings should be at the side or back and there should be no pockets that liquid could get trapped in.
Shoes - should be top-sealed. Never wear wellington boots due to the chance of spillage inside the boots or open sandals, which offer no protection in the event of spillage.
General - Sleeves and trousers should be worn outside gloves and boots. All metallic jewellery should be removed to prevent liquid becoming trapped under them.
5.2 Training
Training should be given in all aspects of the use and handling of cryogenic materials. A combination of on the job skills, instructions and information covering the following areas provides a minimum standard to which all users must be trained.
- Understanding of the Material Safety Data Sheet (MSDS), the risks involved and where to obtain information
- Carrying out a risk assessment (including COSHH)
- Use of PPE
- Handling cryogenic materials
- Moving containers of cryogenic materials (> 1 litre)
- Emergency procedures
- Spillage procedures
And if necessary
- Manual handling of larger storage vessels;
- Dispensing bulk quantities (> 1 litre);
- Vehicular transportation and delivery of cryogenic material.
Much of the training will be carried out as on the job training. This should be done by a competent person (specified by the local School/Unit rules) and an individual training record should be kept for each person handling cryogenic substances.
5.3 Tips on Handling Cryogenic Liquids - DO IT WITH CARE!
- Ensure the vessel is dry and pour cryogenic liquids slowly into the receiving vessel to minimise splashing, spillage and thermal shock to the vessel;
- Use tongs when placing objects into or removing them from cryogenic liquids
- Avoid use of wide-necked, shallow vessels to prevent excessive evaporation and the possibility of oxygen enrichment
- Whenever removing cryogenic liquids from pressurised vacuum insulated vessels (PVIVs) carry out a safety check. A checklist should be situated next to the PVIV for use by trained personnel
- Use an appropriate rod for checking the level of the cryogenic liquid in a dewar
- When removing cell-line cages from storage use a hook to locate the handle and raise the cage
- Never overfill dewars
5.4 Spillage Procedures and Notification
Minor spillage (< 1 litre)
- Allow liquid to evaporate, ensuring adequate ventilation;
- Following return to room temperature, inspect area where spillage has occurred;
- If there is any damage to the floors, benches or walls, report it to Estates and Buildings;
- If any equipment has been damaged following the spillage, inform the relevant person.
Always notify the School/Unit Safety Co-ordinator and complete a University Accident or Near Miss Report Form.
Major spillage (> 1 litre)
- Shut off all sources of ignition;
- Evacuate area of all personnel;
- Inform named person (in the local rules);
- DO NOT return to the area until it has been declared safe by an appropriately qualified person
5.5 Disposal
Care needs to be taken when disposing of cryogenic liquids
DO NOT pour cryogenic liquids down the sink - they will crack waste pipes causing potentially dangerous leaks
DO NOT store cryogenic substances or allow them to vaporise in enclosed areas, including:
Fridges, Cold Rooms, Sealed Rooms and Basements
DO ensure that the area in which the cryogenic liquid is left to vaporise is well ventilated
5.6 First Aid
Burns
- Remove any restrictive clothing - but not any that is frozen to the tissue;
- Flush area with tepid water (not above 40 ºC) to return tissue to normal body temperature;
- DO NOT apply any direct heat or rub affected area;
- Cover with a loose sterile dressing and keep patient warm;
- Obtain medical assistance from the School/Unit First Aider or Ambulance Service
Anoxia
DO NOT attempt to rescue anyone from a confined space if they were working with cryogenic materials and have lost consciousness - call the Fife Fire Brigade and Ambulance Service.
- If someone becomes dizzy or loses consciousness while you are there move them and yourself to a well-ventilated area immediately;
- If breathing stops apply artificial respiration;
- Seek help from School/Unit First Aider;
- Keep casualty warm and at rest;
- If deemed necessary call for an ambulance
Explosions
- If a tube or dewar explodes injuring someone, seek immediate medical attention
5.7 Storage
Installation
For typical laboratory usage follow requirements set out in British Compressed Gases Association (BCGA) Code of Practice 4 (Industrial Gas Cylinder Manifolds & Distribution Pipework/Pipelines (excluding acetylene)). This covers systems carrying oxygen, argon, nitrogen, helium, carbon dioxide, hydrogen, methane, LPG and mixture of these gases.
Storage Vessels
Vessels for storage must be chosen carefully as the properties of many things change at very low temperatures. While most metals become stronger, other materials, such as carbon steel, plastics and rubber, become brittle or even stress fracture at such low temperatures. The vessel must be able to withstand both the temperatures and pressures that it will be exposed to.
Equipment and systems must be kept scrupulously clean to avoid contamination with materials that could be combustible should oxygen enrichment occur
Dewars - purpose designed, non-pressurised vacuum flasks used to store smaller quantities (ca 1-50 litres) of cryogenic liquids. They have loose fitting stoppers to allow boil-off. If any part of a dewar is glass, it should be taped to prevent shattering should an explosion occur. While smaller dewars may be hand-carried, larger ones are moved by purpose designed trolleys.
Pressurised vacuum-insulated vessels (PVIVs) - liquid storage vessels. In most cases these are the property of the supplier. Each is individually marked on the shoulder to identify it and its test history. They vary in size, material composition, mass, stability, etc.
- The PVIV must be identified with a statutory label
- A maintenance record should be kept for each PVIV. Maintenance checks should be carried out on a regular basis by the owner of the PVIV in accordance with BCGA Code of Practice 4.
- Be aware of the safe working life for each item - they will need to be replaced at set intervals
- Whenever a cylinder is delivered, carefully check all aspects (regulator, inlet, outlet, any gauges) for contamination, BS EN numbers and/or CE marks, damage and leaks. If unhappy or uncertain about anything, REJECT IT. Ensure it is adequately labelled to prevent anyone using it and ask the supplier to come and remove it.
- Cylinders should be chained to an anchor point to prevent them falling over.
Bulk storage and dispensing areas
These must:
- display hazard-warning signs to alert people to the presence of ;
- cryogenic liquids;
- be restricted to the relevant personnel only;
- be no-smoking and no naked flame areas;
- be well ventilated, including make-up air;
- have an atmospheric oxygen monitor to detect for and warn about oxygen enrichment or deficiency;
- have a safe means of escape;
- be designed specifically for use (bulk storage areas);
Further information on the storage of cylinders is provided in the BCGA Code of Practice 28 (Vacuum Insulated Tanks of not more than 1,000 litres volume which are Static Installations at User Premises : 1997).
Ventilation and Alarm Requirements for Bulk Storage and Dispensing Areas
In any room where cryogenic liquids are going to be stored, the equation given in Annexe III should be used to assess whether maximum spillage of the cryogenic liquid would result in a dangerous decrease in atmospheric oxygen. Where it is established that oxygen deficiency could occur advice should be sought from the University Safety Adviser.
5.8 Transportation
When transporting dewars the following aspects should be taken into consideration:
- The correct personal protective equipment to be worn;
- Is the destination ready to accept it?
- Does the route take you through populated work areas?
- Are there any slip or trip hazards (including stairs) which could result in spillage?
- If transported on a trolley, is the route passable (steps, kerbs)?
- Is the dewar going to be transported in a lift?
NEVER travel in a lift with a dewar
The transportation of liquid nitrogen in dewars is covered in the BCGA Code of Practice 30 (The Safe Use of Liquid Nitrogen Dewars up to 50 Litres : 2000).
Transportation of liquid helium requires special attention, as the containers in which it is transported are specialised and relatively fragile
Transportation by Lift
- DO NOT travel in the lift with the dewar
- One person places the dewar in the lift while another waits to receive the dewar from the lift once the journey is complete
- Use key controlled lifts whenever possible
- Make sure that there is a clearly visible sign on the dewar warning others not to enter the lift with the dewar
A lift is a confined space and should leakage of the cryogenic substance occur, anoxia or asphyxiation is a potential danger.
Handling Cylinders
- Should only be carried out be trained personnel
- Specially designed trolleys should be used
- Use the appropriate personal protective equipment
- Use the Material Safety Data Sheet to establish the substance being handled
- Plan and check the route to be taken
NEVER
- Turn your back on a free-standing cylinder
- Attempt to catch a falling cylinder - get out of the way!
- Roll cylinders along the floor on their sides
- Handle cylinders alone
- Travel in a lift with a cylinder
- Transport more than 1 litre of cryogenic liquid by yourself
Handling cylinders comes under the Manual Handling Operations Regulations 1992. The application of these in regard to cylinders is covered in the BCGA publication GN3 : The Applications of the Manual Handling Operations Regulations to Cylinder Handling.
Vehicular Transportation of Cryogenic Materials
Transportation of cryogenic substances is covered by the Carriage of Dangerous Goods by Road (CDG Road) Regulations 1996. These Regulations cover specific volume/mass of dangerous goods that may be transported, duties of responsibility, correct packaging and labelling of goods, vehicle usage and driver training.
Note: - These Regulations come into force if the cryogenic substances are transported on public roads, for however brief a period.
University Vehicles (Dewars < 25 litres - load < 500 litres)
- Written risk assessment for the activity;
- Appropriate label on container describing the contents;
- Vehicle suitable for the purpose (driver separated from load by bulkhead);
- Driver made fully aware of the nature of load, associated hazards and emergency procedures;
- Appropriate PPE provided to driver;
- An information sheet carried within the vehicle to inform the Emergency Services of foreseeable hazards (see Annexe VI).
Third Party Vehicles (Dewar > 25 litres)
The transport company should be supplied with the following information:
- Product designation, i.e. NITROGEN, REFRIGERATED LIQUID
- Product classification code, i.e. Class 2.2.
- Product UN number i.e. UN 1977
- The volume of each dewar and the number of dewars
- The consignor's name and address
- The address of the consignee
- A statement signed or authenticated by, or on behalf of, the consignor confirming that in accordance with the relevant provisions of the Carriage of Dangerous Goods by Road Regulations 1996 and the Carriage of Dangerous Goods (Classification, Packaging, Labelling and Provision of Information) Regulations 1996.
- the dangerous goods as present may be carried
- the dewars are properly labelled
Transport of Large Volumes (> 500 litres)
If the total volume of cryogenic liquid being transported is in excess of 500 litres, the Carriage of Dangerous Goods by Road Regulations 1996 are applicable in full. If the total volume being transported is in excess of 500 litres and the dewars are being transported in a vehicle over 3.5 tonnes, the Carriage of Dangerous Goods by Road (Driver Training) Regulations 1996 are applicable. As it is unusual for users to transport such volumes, the requirements of these regulations are not covered by this document.
For further guidance contact the University Safety Adviser on Ext. 2751.
ANNEXE I
Physiological Effects and Recommended Exposure Limits of Nitrogen
Asphyxia due to oxygen deficiency is often rapid with no prior warning to the victim. A general indication of what is liable to happen is given in the table below but it should be appreciated that the reactions of some individuals can be very different from those shown.
Effects and Symptoms
Oxygen Content (Vol %) (at atmospheric pressure)11-14 :
- Diminution of physical and intellectual performance without person's knowledge
Oxygen Content (Vol %) (at atmospheric pressure) 8 - 11:
- Possibility of fainting after a short period without prior warning
Oxygen Content (Vol %) (at atmospheric pressure) 6 - 8:
- Fainting within a few minutes; resuscitation possible if carried out immediately
Oxygen Content (Vol %) (at atmospheric pressure) 0 - 6:
- Fainting almost immediate death ensues;brain damage even if rescued
ANNEXE II
Guidance for Assessment of Ventilation Requirements
NOTE: (This section is taken from the Guidance entitled 'Code of Practice CP30 - The Safe Use of Liquid Nitrogen Dewars up to 50 Litres' and is published with the permission of the British Compressed Gases Association (BCGA) ).
The type of ventilation depends on a multitude of factors such as the type of location, gas type, possible leaks etc. .
Ventilation can be natural or provided by forced ventilation. The design criterion is the number of air changes per hour.
In locations above ground level with no special ventilation openings, natural ventilation provides typically 1 air change per hour. This is not the case in buildings with windows sealed with tight seals. For underground rooms with small windows, 0.4 air changes per hour can be considered an average value.
For handling (storing, filling, withdrawal etc.) transportable cryogenic vessels with non-flammable non-toxic contents in locations above ground level, natural ventilation is generally sufficient, provided the room is large enough and the outdoor area is not enclosed by walls etc. .
An indoor location should have ventilation openings with a total area of 1 % of the ground area. The openings should be positioned diagonally across the room. The density of the gas should also be taken into consideration (the main opening at the highest point for gases lighter than air, or at ground level for gases heavier than air).
For more than 2 air changes per hour, a vforced ventilation system is necessary. Different situations may require a specific number of air changes per hour e.g. 5, 10, 20 etc. .
In typical situations the number of air changes can be calculated, assuming a certain leakage rate from the vessel and a homogenous distribution of gas using the formula:
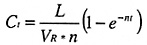
Ct = Gas concentration
L = Gas release (m3/hr)
VR = Room volume (m3)
n = air changes per hour
t = time in hours
* = multiple
For long periods (t tending to infinity):
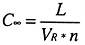
Cinf = Gas concentration after a long period
A worked example of this is in Annex IV, showing how to assess whether natural ventilation rates can be adequate for real situations.
ANNEXE III
Method of Calculating the Potential Oxygen Depletion in a Room Due to to Liquid Nitrogen Filling and Spillage
This annex considers three scenarios - filling losses which always occur when the Dewar is being filled, spillage of the contents of the Dewar and the 'Worst Case' scenario where the entire contents of the vessel are lost to the room immediately the Dewar is filled.
The resulting oxygen concentration in the room may be calculated from the following formula for each of the scenarios:
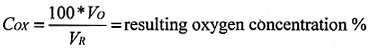
where:
V0 = The volume of oxygen (m3)
VR = The room volume (m3)
* = multiply
V0 is calculated for the following scenarios:
Filling
A value of 10% of the volume of the product in the dewar is used to estimate the losses to atmosphere during filling:
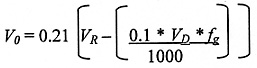
where:
0.1 = 10% volume loss during filling
VR = The room volume (m3)
VD = Dewar capacity (Litres)
fg = Gas Factor. This is 683 for nitrogen (Nitrogen gas takes up 683 times the volume of liquid nitrogen, i.e. one litre of liquid nitrogen 683 litres of gaseous nitrogen.).
0.21 = The normal concentration of oxygen in air (21%)
* = multiply
Spillage
For the spillage of the entire contents of a liquid nitrogen Dewar:
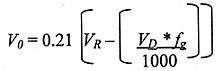
where the symbols are as before.
Filling and Spillage Together
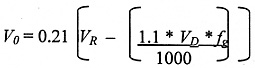
The 'Worst Case' scenario, where the entire contents of the Dewar are lost into the room immediately after filling, equivalent to 110 % of the vessel contents to allow for 10% filling losses prior to spillage:
1.1 = 110% volume loss during filling
VR = The room volume (m3)
VD = Dewar capacity (Litres)
fg = Gas Factor. This is 683 for nitrogen (Nitrogen gas takes up 683 times the volume of liquid nitrogen, i.e. one litre of liquid nitrogen 683 litres of gaseous nitrogen.).
0.21 = The normal concentration of oxygen in air (21%)
* = multiply
NOTE: Risk Assessments must assume the 'Worst Case' scenario of spillage after filling.
A worked example of this is shown in Annex IV
ANNEXE IV
OXYGEN DEPLETION EXAMPLE
Example:
A basement room contains two 25 litre and three 10 litre dewars:
Room dimensions = 7 x 8 x 2.5 metres = 140 m3
25 litre dewar - loses 0.2 litres per day through evaporation
10 litre dewar - loses 0.15 litres per day through evaporation
(Dewar's manufacturers quoted evaporation rates).
Normal Evaporation Losses
Evaporation is a continuous process, hence the increase in nitrogen concentration (Ct) can be calculated over a long period using:
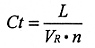
where:
L = gas evporation rate (m3/hour)
VR = The room volume (m3)
n = air changes per hour
* = multiply
Whilst manufacturers will quote the evporation rate for their dewar, it is prudent to double it when calculating the rate of nitrogen release L. This allows for a deterioration in the insulation performance over the lifetime of the dewar. The nitrogen gas factor of 683 has to be used to calculate the volume of gaseous nitrogen released through evaporation, as the dewar manufacturer's figures relate to the volume of liquid nitrogen lost.
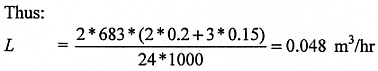
Assume there is an average of 0.4 air changes per hour in the room. The nitrogen concentration increase is, therefore:
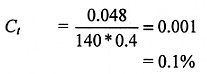
Air already contains 78% nitrogen; thus, in this case, evaporation from the five dewars in the circumstances described would reduce the oxygen concentration by some 0.02%. This is because air contains 21% oxygen, so the oxygen depletion can be approximated as 0.1% x 0.21 = 0.02%.
In this example, normal nitrogen evapouration from the dewars has only a small effect in increasing the nitrogen concentration, and thus reducing the oxygen concentration, in the room. If, however, far more dewars were stored in the same room used in the above example, or in a much smaller room was used for the five dewars mentioned, then the nitrogen concentration would increase by a much larger factor. If Ct in such a case was calculated to be 0.05 (i.e. 5%), then forced ventilation would be recommended, since this would reduce the oxygen concentration in the room by 1%, which is at a level where the safety margin has been virtually used up.
Losses Due to Filling
First calculate the volume of oxygen in the room, Vo .
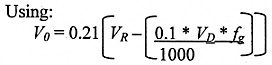
where:
VD = capacity of largest dewar (litres)
VR = The room volume (m3)
fg = Gas Factor. This is 683 for nitrogen (Nitrogen gas takes up 683 times the volume of liquid nitrogen, i.e. one litre of liquid nitrogen 683 litres of gaseous nitrogen.).
* = multiply
The same dewars and room size are used (140 m3), but the largets nitrogen release is during the filling of the largest dewar (25 litre) dewar and again the nitrogen factor of 683 must be used to convert liquid to gaseous nitrogen.
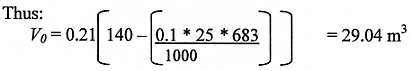
The resulting oxygen concentration in the room (Cox) can then be calculated:
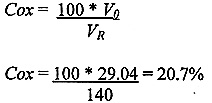
Clearly, this is no problem. As a guide it is recommended that the combined effect of normal evaporation and filling processes should give rise to alarm if the oxygen concentration level falls below 19.5%.
Losses due to Filling and Spillage
Following the same process as above, calculate the volume of oxygen in the room (Vo ) as a result of the spillage of the entire contents following filling. Annex 3 shows that the factor of 1.1 is used to allow for filling losses, plus the total loss from spillage.
Using:
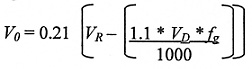
VD = capacity of dewar (25 litres)
VR = The room volume (140 m3)
fg = Gas Factor. This is 683 for nitrogen (Nitrogen gas takes up 683 times the volume of liquid nitrogen, i.e. one litre of liquid nitrogen 683 litres of gaseous nitrogen.).
* = multiply
Again we have a 140 m3 room and again the largest release is from the 25 litre dewar.
Thus:
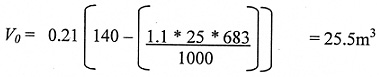
Then calculate the resulting oxygen concentration (Cox) after spillage:
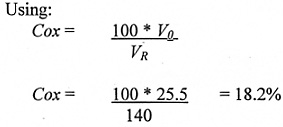
This is just above the level (set at 18%) at which we recommend the oxygen monitor should give an emergency alarm, leading to immediate evacuation.
In this example, we would recommend an oxygen monitor be fitted with two levels of alarm:
- 19.5% should lead to urgent investigation and corrective action;
- 18.0% should cause immediate evacuation - assuming that this level results from spillage, follow the actions described in this guidance.
ANNEXE V
Cryogenic Storage Checklist
This checklist should be completed by the School/Unit Safety Co-ordinator for each location where bulk quantities of cryogenic materials are stored. Should the answer to any of the questions be NO, an action plan is required.
School/Unit ............................................................................................
Store Location ......................................................................................
Cryogenic Material .............................................................................
- Does the room have mandatory safety warning signs on the door?
- Is suitable PPE provided?
- Are there maintenance records for the:
a). Storage equipment (cylinders, regulators),
b). Ventilation equipment?
c) PPE - Is there adequate ventilation?
State ventilation: .............................................................................................(e.g. Mechanical, natural etc.)
Number of air changes per hour:
a) normal cycle ................................................./hour
b) emergency cycle ........................................../hour - Is there a warning device in case of:
a) oxygen enrichment/deficiency?
b) failure of ventilation? - Is there a written emergency protocol?
- Are there written standard operating procedures in place for handling cryogenic materials?
- Is the room restricted to trained users?
- Have all users been given copies of the standard operating procedures and emergency procedures?
- Has the atmospheric oxygen shift following maximum spillage been determined?
- Has a Registration of Bulk Cryogenic Storage and Dispensing Areas form been completed and returned?
- Is there a designated contact person in the case of emergencies? ..
Signed ................................................................................. Date ....................................................
Print Name .......................................................................... Position ...............................................
ANNEXE VI
Small Volume Users Checklist
Material ....................................................................
Volume .....................................................................
Has a risk Assessment been performed?
Personal Protective Equipment (PPE) to be worn:
Gloves
Face Visor
Laboratory Coat / Overall
Covered Shoes
Other (Specify).
Between the collection point and the destination are there any of the following?:
Stairs
Lift
(REMEMBER - Never travel in a lift with a dewar)
.
Procedure following accidental spillage of a cryogenic material:
....................................................................................................
....................................................................................................
Make sure that the cryogenic material is not allowed to evporate in an enclosed area. This includes:
- Fridges;
- Cold Rooms;
- Sealed Rooms;
- Basements.
Areas where cryogenic materials are kept MUST be well ventilated.
ANNEXE VII
TRANSPORT EMERGENCY CARD (To be complete by the consignor)
Cargo:
Nature of Hazard:
Protective Devices:
_____________________________________________________________
EMERGENCY ACTION
- Stop the engine
- No naked lights. No smoking
- Warn other road users
- Keep public away from the danger area
- Put on protective clothing if appropriate
SPILLAGE
- Contain leaking liquid with absorbent material e.g. dry sand
- Prevent liquid entering the sewers or basements as vapour may create toxic atmosphere
FIRE
- If safe to do so - attack fire with a dry powder extinguisher
FIRST AID
- If substance has got into eyes immediately wash out for several minutes
- Remove contaminated clothing immediately and wash affected skin with plenty of water
- Seek medical treatment when anyone has symptoms apparently due to inhalation, swallowing, contact with skin or eyes, or fumes produced in a fire
- Even if there are no symptoms, send to a doctor and show him this:
______________________________________________________________________
Additional information provided by consignor
______________________________________________________________________
TELEPHONE______________________________________________________________________
WARNING: This information is intended to assist any person involved in recognising the characteristics of this load. It is emphasised that such substances may vary according to conditions; this information, therefore must be regarded as a general guide.
ANNEXE VIII
EMERGENCY ACTION PLAN
MAJOR RELEASE OF TOXIC GAS,
LOW TEMPERATURE LIQUEFIED GAS
or
MAJOR SPILLAGE OF HAZARDOUS SUBSTANCE
- Evacuate the room or, if considered necessary, the building;
- Control access to the room/building
- DO NOT ATTEMPT TO RESCUE A COLLAPSED PERSON
or
COMMENCE A CLEAN-UP OPERATION
UNLESS YOU ARE CONFIDENT THAT THIS CAN BE CARRIED OUT WITHOUT RISK - Call the Fire Brigade: Dial 9999 (University Telephone System)
Ask for the Fire Brigade and state precise location and nature of incident.
Wait for confirmation of message. - Inform the Safety Co-ordinator
- Ensure that an appropriate person is delegated to meet the Fire Brigade and provide any additional information which they may require, e.g. the exact location of the accident and any specific hazards to which firemen may foreseeably be exposed.
- DO NOT RETURN TO THE ROOM OR THE BUILDING UNLESS AUTHORISED TO DO SO BYTHE SENIOR FIRE OFFICER PRESENT
- Ensure that all occupants of the room/building and other relevant persons e.g. janitorial, trades or contract staff, particularly those who work outside normal hours, are made aware of any restriction imposed on entry to the building or a particular area/room.
Environmental, Health and Safety Services
August 2000
